AseptiScope Partners with Protolabs to Limit Pathogens in Clinical Settings
San Diego company develops unique stethoscope cover system that limits disease transmission.
AseptiScope is a San Diego-based start-up company whose mission is to protect clinicians and patients through novel health care devices. Their flagship device is a touch-free system that combats a common means of spreading germs within doctor's offices and other clinical environments.
Known as the clinician's third hand, the stethoscope—in one form or another—has been a staple tool of the medical profession since the 1860s. Improvements to the original design took it from a tube form to today's binaural design—for use in two ears. And that's pretty much where we stand today, with the inevitable physical contact between the patient and a potentially microbe-covered device. Previous standards required clinicians to wash their hands and use alcohol wipes to kill germs that could be transferred from person to person, but that only got rid of a portion of the pathogens that could be present in a busy clinical setting.
Problem: Disrupting the Pathogen Pathway
Recent CDC draft guidelines go a step further to prevent transmission, and AseptiScope—a start-up in San Diego—has developed a device to address those requirements.
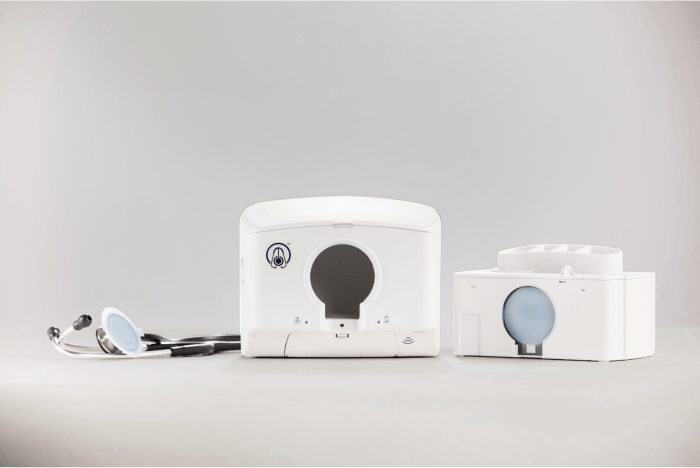
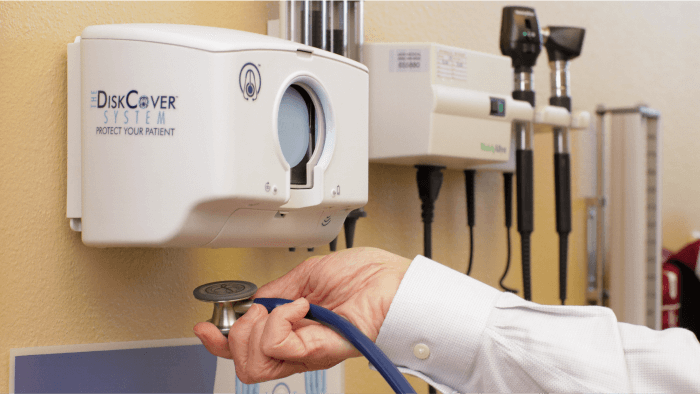
The DiskCover System offers a no-contact, one-time use cover for a stethoscope's diaphragm (the part that's placed on a patient's body). A single cartridge holds 420 covers on a spool. When clinicians wave a stethoscope beneath the device, a new cover is wound forward and displayed, allowing clinicians to place their stethoscopes in a window where the new cover transfers to the diaphragm. Once used, the cover can be removed via a peel tab and recycled.
A counter maintains usage data, ensuring compliance, and lets staff know when it's time to change a cartridge. It's a remarkably simple concept, but with high design requirements.
"There's never been a real touch-free delivery system with a functional intermediary component that facilitates a clean transfer and lets you ship hundreds of these products to hospitals," said Scott Mader, AseptiScope co-founder and CEO. "You can easily pop them into an already-established process and collect information while you're providing a completely clean technology."
The idea seems simple but there's a lot to it, and it required multiple iterations to get it just right.
Solution: The Parts
Three of the four parts Protolabs manufactured for The DiskCover System make up elements of the internal cartridge, called the Clean Cassette. These critical parts fit into the body of the Clean Cassette and hold a large spool that contains the covers. When a cover is used, the backing tape liner rolls forward onto an empty spool, much like a VCR—except this "tape" moves right to left.
Because the tape must move freely and accurately through the path, precision moulding was important to the AseptiScope team. If the outward-facing adhesive cover were to stick to the pathway, there would be little customer satisfaction. "It's not an easy thing to do when there are a lot of narrow crevices and internal ribs and you need draft," said Kelly Powers, AseptiScope co-founder and COO.
The Clean Cassette cartridges can be replaced by clinical staff when the spool runs out of disk covers. Both Mader and Powers referred to the cartridge as their razor blade—the core component of The DiskCover System.
In need of applications engineering expertise, AseptiScope partnered with Protolabs consultative design services to refine the moulds for the lid and base of the cartridge multiple times.
"We had five major iterations of the cartridges and multiple smaller ones along the way. We had to get it right," said Powers. "Protolabs was very creative with us, letting us know, 'You can't have that rib internally stand up straight like that, or you have to have draft on that.' It took a lot of precision, detail, and diligence." In the design, six snaps on the lid must be able to come together perfectly for it to be a closed system.
A very critical but small area required that Clean Cassette cartridges should snap into an area holding an authentication chip. That had to be perfect. "To check sizing, we basically backed away all the other features around the region and 3D printed it," said Kevin DelSignore, AseptiScope Senior Director of Engineering and Quality.
They used our extremely high-resolution 3D printing option as a prototyping step, testing the snaps with MicroFine™ material in an orientation that supported the layers in a way that was close to what DelSignore expected with a mould feature. MicroFine™-based stereolithography (SLA) offers feature sizes as small as 0.127mm and layers as thin as 0.0254mm. Using 3D printing allowed for faster turnaround, speeding development of the part, which measured 22.02mm x 6.13mm x 11.18mm
Two other parts: a take-up spool for the tape liner and a mounting bracket for the dispensing system were also moulded.
ABS plastic was the material of choice for the final parts as they had to survive a drop test to ensure being knocked about by mobile IV poles, beds, and other potential hazards in the clinical setting. ABS is a high-impact resistant, high-gloss material that meets UL 94 HB flammability ratings.
The AseptiScope team had concerns with sending the technology overseas due to shipping costs/delays and keeping the IP in country. Initially the team worked with a local manufacturer but moved to Protolabs for design modifications and production quantities. The availability of rapid tooling and unlimited moulding was a major factor in the decision to go with Protolabs.
Outcome: To Market
The DiskCover System is available and the AseptiScope team expects a high ramp-up in production and sales following the official release of the new CDC guidelines. They have already established recycling services if customers return the Clean Cassettes to the company, although many hospitals already have recycling programs in place. This compares to companies offering $6 (£4.59) disposable one-use stethoscopes, which are merely landfill fodder.
Although it's early in the lifecycle of this product, they are already envisioning updates in the future to make The DiskCover System even more robust, including the possibility of changes to the mounting structure to offer more options. Other products are on their whiteboards, as well.
One of the company's learnings from the development process was how using a domestic manufacturer benefitted them, as did the free expertise offered via our consultative design services. As Powers summed it up: "Don't underestimate or be dismissive of the advantages of early engagement with your injection moulders... [working even sooner with Protolabs] would have saved us a lot more time and stress."
| At A Glance |
|---|
|
It is well-known in the medical community that pathogens can move between clinicians and patients via stethoscopes, probably the most used medical tool. Research indicated that existing practices were insufficient for killing germs, especially ones that are highly resistant, such as C. diff. and MERSA. What was needed was a simple-to-use and easily adopted solution to create a protective barrier in the link between clinician, stethoscope, and patient AseptiScope's flagship product, the DiskCover System, places an aseptic disposable barrier (disk cover) onto the diaphragm of the stethoscope. The simple concept became reality as AseptiScope teamed up with Protolabs to simplify and improve an already good design for the device, assuring that it was touch-free and rugged enough to endure the bumps and jarring inherent in many clinical settings. It also had to ensure an aseptic environment to avoid contamination during use. Along the way, the company used micro-resolution 3D printing on one of the parts to test for fit and function before injection moulding all the parts at production volumes.
Leveraging Protolabs' consultative design services for injection moulding and unlimited on-demand manufacturing moulds, development moved quickly and the DiskCover System became available well ahead of the soon-to-be-adopted Center for Disease Control (CDC) guidelines. |





