Prototyping and Low-Volume Production for Medical Applications
How rapid manufacturing can increase speed to market for devices and components
Developing parts and devices for the medical industry is a challenging, high-stakes process where speed (time to market) and accuracy are critical to success. Evaluating new product designs and materials; iterating those designs fast when needed; and selecting suppliers that can quickly, consistently and cost-effectively provide prototypes and low-volume production, are important factors in attaining this success.
This white paper explores injection molding, CNC machining and 3D printing, and weighs each technology’s strengths and weaknesses relative to prototyping and low-volume production for the medical industry. It also discusses the product development and the Food and Drug Administration (FDA) approval process, including verification and validation, and form and fit testing.
What to Look for in a Prototyping and Low-Volume Manufacturer
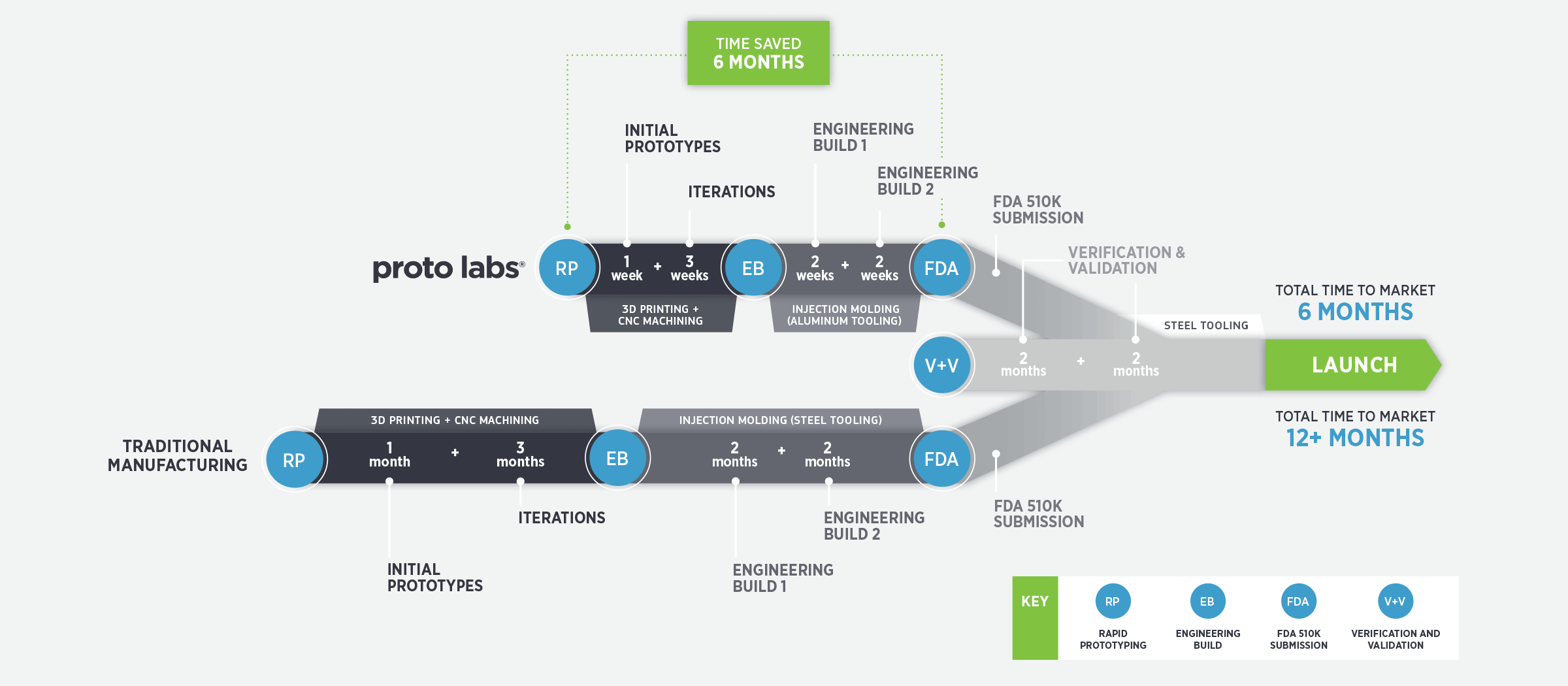
The medical product development process is all about successfully speeding through the FDA gates, so a manufacturer that can quickly provide quality prototype parts is critical. One that has excess production capacity won’t be swamped by a large, rush order that could add unnecessary time to the process. A manufacturer that has successfully removed inefficiencies should be able to provide prototype parts in a few days, not months.
The manufacturer you select should be fast enough to allow multiple iterations in a condensed time frame, and possess the scale to allow for multiple iterations at the same time, particularly for injection molding. Competitively priced injection molding enable cost-effective short runs so product developers have more time to react with shorter lead times.
In addition to quick turnaround, look for a manufacturer that offers fast, automated quoting so you can get parts as soon as possible. Time is certainly saved when you can upload a 3D CAD model and receive an automated quote within a few hours.
Look for manufacturers that provide immediate design feedback to improve the manufacturability of the parts when the quote is delivered. This drastically accelerates the process, results in better and easier-to-manufacture products, and removes potential visual or performance defects before the first parts are produced.
Always use an experienced manufacturer that has an excellent reputation and track record of providing fast, consistent and quality prototype parts on time. Also, do they have the ability to execute what you ask every time? Do they have a mechanism to apply lessons learned further down the development stage? Do they possess scale to do on-demand orders? Can they closely replicate end production manufacturing methods? Do they have materials used in the medical industry and a basic understanding of medical approval processes? If not, you may be using the wrong manufacturer.

Industrial 3D Printing for Form and Fit Testing
Additive manufacturing, used interchangeably with 3D printing, is an excellent way to quickly evaluate new product designs without making compromises due to complex part geometries, and at low cost compared to tooling costs. Design changes are relatively easy to make and low cost.
Disadvantages of 3D printing for prototyping are higher piece part costs, limited color and texture choices, and in certain instances, plastic-like materials will differ from the final production material used in process like molding and machining. If the surface finish, texture, color and coefficient of friction vary from the end material, it is difficult to accurately assess the subtle needs and benefits of these properties.
The largest advantage 3D printing provides is accurate form and fit testing as the build process of additive technology can accurately produce the form and size of the desired part, making it very useful for early evaluation of new medical parts. Typically, a medical device developer will use 3D printing to identify design flaws, make changes and then make second-generation machined parts or invest in tooling to create injection-molded parts.
Many materials can be 3D printed, however, regardless of the material used, the manufacturer of the finished good is responsible for ensuring the safety and efficacy of the parts and materials used, and they must be tested to be shown safe in the application and environment in which they will be used.
Implantable parts and devices must meet a higher standard of biocompatibility, and all risks to the patient from the materials used need to be understood.
Stereolithography (SL) is one of the more frequently employed additive technologies. It’s regularly used for producing models, prototypes, patterns and, on occasion, production parts. The process involves building a part (or even a multi-part device as one) in single, thin layers by curing a photo-reactive resin with a UV laser or similar power source. SL is commonly used to create prototype parts in clear materials in order to view the flow of liquids through the parts. Some other processes may result in porosity or surface roughness that could impair any desired watertight properties. To aid this process, it’s important to select materials that resist water absorption and are formulated to be clear. For example, Accura ClearVue plastic is designed to produce virtually colorless parts for a range of applications.
For form and fit evaluation of devices, high-resolution stereolithography has greatly expanded applications for medical devices/parts. Rapid prototyping using SL enables nearly effortless design iteration for form and fit. In addition to accelerating this process, 3D printing allows product designers to focus on the role the part needs to perform, relying on the part’s strength, or the shape and fit with minimal reliance on the material’s properties.
Selective laser sintering (SLS) is an additive manufacturing process that uses a CO2 laser to draw onto a hot bed of thermoplastic powder. The laser lightly sinters, or fuses, the powder into a solid. After each layer, a roller deposits a fresh layer of powder on top of the bed and the process is repeated. SLS uses thermoplastic nylons, so parts are accurate and exhibit excellent toughness, however they have a rough surface and lack fine details. SLS can be used to create tough, durable medical prototypes.
Direct metal laser sintering (DMLS) is another additive manufacturing technique used in medical part and device prototyping. The process uses a laser to sinter powdered metal to form fully dense metal parts such as stainless steel and titanium components commonly used in medical devices.
When selecting a 3D printing part supplier, look for an organization that has an excellent reputation for accuracy, quality, speed and reliability to ensure you’ll receive good quality parts as fast as possible.
CNC Machining for Functional Testing
CNC machining starts with a block of material before a tool removes material to create a part. This prototyping method often plays a big role in the early and end-of-life stages of a product’s life cycle because there is no tooling expense and products can be supplied quickly and relatively inexpensively.
Machining enables those developing medical devices to test a number of different part designs in parallel, which in turn, shrinks their development cycle. This upfront, simultaneous testing of prototypes with the same product strength and density as the final product helps device developers be better prepared for passage through FDA gates.
Because device developers can’t merely pivot if they fail to pass a gate—they need to start over—testing multiple design options at each gate provides a greater chance of success to quickly emerge at the end of the process with the right product.
Machining is also important for testing parts and devices with engineering-grade materials. Machined metals and plastics can provide the same in-hand feel and weight as the final product, eliminating false information from doctor testing. For any metal prototyping, CNC machining is usually the best option for testing the product’s strength and weight.
At the end of a device’s life, when demand begins to dwindle, CNC machining can play a cost-effective role to satisfy a need for only a small run of parts. This on-demand production also limits unnecessary warehousing space for excessive product and the financial risks associated with that.
Depending on the complexity of the part, CNC machining can be a cost-effective option for production volumes fewer than 200. For more than 200 parts or if design changes are possible before all testing is complete, creating aluminum tooling for injection molding makes sense over using CNC machining.
Injection Molding for Low-Volume Production
Injection molding is a common production method during medical part and device development, and can provide production parts made from plastic, metal, and liquid silicone rubber (LSR) materials as early as possible—an important benefit during the FDA gating process. This robust process provides repeatable, reliable and consistent results. For most injection molding manufacturers, low volume is considered to be approximately 10,000 to 50,000 parts.
The disadvantages of injection molding at low volumes are the upfront tool cost spread over a low number of parts, and the risk that design changes may be necessary after a tool is made, which can be costly. However, using low-cost aluminum tooling versus expensive steel ($50,000 or more), can reduce financial risk of the initial tooling investment.
It’s important to understand some common shortcomings of injection-molded parts. Some flash, parting lines, weld lines, knit lines, gate marks and ejector pin marks typically are present on the final part. Extremely challenging or poorly designed injection molded parts will likely cause challenges later. Parts designed to the best injection molded standards and practices will provide the largest operating window to produce good parts.

Plastic, Metal, and Liquid Silicone Rubber Materials
Device developers have a number of plastic, metal, and liquid silicone rubber materials, in various grades, available to them during prototyping and low-volume production, and each provides different properties and application opportunities.
Due to the sterilization need for medical devices, ensuring a material can withstand autoclave, E-beam or gamma sterilization is extremely important. Material biocompatibility is also critical in facilitating healthy interaction for devices that encounter skin contact with patients. PEEK and PEI are two plastic materials with long-term biocompatibility that withstand steam autoclaving while retaining their rugged physical properties.
USP Class VI and ISO 10993 tests are designed to assess the biological reactivity of various types of plastic materials in vivo (inside the body) and while this testing is not a substitute for biocompatibility testing, it often is used by manufacturers to classify materials. Many plastics suppliers find it advantageous to have their resins certified as USP Class VI and ISO 10993, especially if the resin is likely to be used in medical devices. A plastic resin that has passed these certifications is assumed to be more likely to produce favorable biocompatibility results.
There are numerous metals that can be used to prototype parts via CNC machining and 3D printing processes as well as metal injection molding (MIM) for increased volumes. Protolabs, for example, has more than 30 hard and soft metals that can be machined, a handful of available additive metals such as cobalt chrome, Inconel and titanium, and stainless steel and nickel steel used for injection molding. A particularly hard, tough and strong stainless steel, 17-4 PH stainless steel is useful for medical instruments. Liquid silicone rubber is a regular material found in medical products due to its thermal, chemical and electrical resistance; its biocompatibility; and its ability to retain its properties at extreme temperatures during sterilization. LSR molding is a common manufacturing process used to achieve quality LSR parts, which can manufactured in low volumes in a few weeks at suppliers like Protolabs.
Overmolding—molding a soft, flexible part on the outside or inside of a hard part—is growing in the medical industry and often is more cost effective than assembling multiple components. The overmolded material, such as an elastomer or a liquid silicone rubber, must be easy to clean and should be considered as one part in the design. A surgical device with a rubber overmolded handle is a good example.
There are advantages to using a clear material in prototyping to view the flow of liquids and gain a greater understanding of how everything works together. Parts may be less cosmetically appealing, but if it’s prototyping purpose is to monitor fluid interaction inside the part, cosmetics can be addressed in future iterations.
Before attempting to create a watertight, two-part sealed component, determine how you intend to seal it. Does the design allow ultrasonic welding? This can scratch and damage parts, especially clear parts. Hot plate welding is less desirable but the more challenging process may be selected due to fewer cosmetic constraints like scratching.
A method to track iterations that is repeatable and has a low risk of failure or human error should be developed e.g., using different color-coded parts to track iterations. Note that major color changes can create molding issues, e.g., splays and knit lines can vary by color.
End-use medical products need to be produced from a medically certified material and manufactured in an appropriate environment, such as a clean room for some parts. Due to a longer development cycle, it’s important to select a material that won’t be discontinued during the expected production life of the product, or a manufacturer will need to re-pass gates to prove a new material works. Choose materials that don’t have availability issues that could disrupt manufacturing schedules.
Moving Devices to Market Launch
Once a company decides to develop a new medical device and subsequently creates a business plan, it must classify the device based on the risk to the patient, as lowest risk (e.g., toothbrush), moderate risk (blood pressure cuff) or highest risk (stent). Depending on where the device will ultimately be used, the appropriate premarket submission must be prepared.
A 510(k) is a premarket submission made to the FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent, to a legally marketed device that is not subject to premarket approval (PMA). 510(k) premarket notification to the FDA is required at least 90 days before marketing unless the device is exempt from 510(k) requirements.
Premarket approval is the most stringent type of device marketing application required by the FDA, according to the organization. A PMA is an application submitted to FDA to request approval to market. Unlike premarket notification, “PMA approval is to be based on a determination by FDA that the PMA contains sufficient valid scientific evidence that provides reasonable assurance that the device is safe and effective for its intended use.”
The Institutional Review Board (IRB) “reviews research projects that involve human subjects to uphold two broad standards: first, that subjects are not placed at undue risk; second, that they give uncoerced, informed consent to their participation.” Approval of the IRB is necessary to conduct pre-clinical trials to test the safety of things that interact with patients.
The FDA generally recognizes stage gate or waterfall processes to ensure an organization creates product designs that meet user needs and product requirements. Many consulting and industrial design firms are familiar with the FDA approval process and can act as consultants to organizations looking to navigate this process.
“Companies need to be able to quantifiably test product requirements against user requirements,” says Paul Jossart, manufacturing manager for Nova-Tech Engineering. “Once firmly documented user needs and design requirements of the device to meet those needs are established, it’s handed off to a design team for electronic modeling and ultimately prototyping.”

Verification, Validation, and Qualifications
Verification varies by product, part and classification, and aims to ensure that a product or part is produced in an appropriate, consistent and repeatable manner and also meets the design intent. Validation aims to ensure a product or part meets all the design specifications as well as customer needs.
Installation Qualification (IQ) verifies that equipment has been safely and correctly installed to meet the manufacturer’s requirements. Manufacturer-prescribed tests are then performed and the results evaluated to ensure compliance with those requirements. Operational Qualification (OQ) is a testing process to ensure equipment functions properly to meet test plan specifications. If the results don’t meet specifications, adjustments must be made and recorded, and testing is performed again. The results are documented in a written report. The purpose of Performance Qualification (PQ) of equipment is to validate the production process and ensure its results can be replicated under typical working conditions.

Improving Probability of Success
“It’s important to get the types of materials that will be used in the final product—and particularly materials that interact with the patient—as soon as possible in the testing phase,” says Jossart. “The manufacturer must be able to make the device to meet the design intent so they know the device will perform satisfactory later.”
The faster a manufacturer can get product prototypes into an engineer’s hands, the faster the device can get to market. Because the physics of materials vary, matching the characteristics of the final product material is important in order to get valid test results.
“Product design and process need to work together for successful product development,” says Jossart. “Hardly anyone ever gets it right the first time. I don’t know how you’d do it without rapid prototyping.
The ability to find a prototyping supplier that can quickly turn low-volume manufacturing parts is critical to success.”
Failing fast, learning what doesn’t work as soon as possible, then working to iterate quickly, is important to passing the FDA gates without having to go back and re-pass them. The right manufacturer can help you do that, moving your device one step closer to market launch.





