
Digital Manufacturing Helps Medtech Firm's Goal to Improve Patient Outcomes
HemoSonics used CNC machining, injection molding, and other services to quickly develop blood analysis machine
As often happens in the medical industry, innovative ideas, hatched in university research settings, spawn innovative companies, which create innovative products. A case in point: HemoSonics.
The Charlottesville, Virginia-based medical device company was started in 2005 by two professors and a post-doctoral research student at the University of Virginia School of Medicine’s Bio-Medical Engineering program—Bill Walker, Mike Lawrence, and Francesco Viola respectively. The trio identified a method for measuring the stiffness of blood clots by using ultra-sound imaging technology, and created a system built around that technology aimed to improve patient outcomes and reduce costs.
A number of years followed of extensive research and development, which included securing key patents, conducting numerous hospital studies, and consulting with physicians and other clinicians.
More recently, HemoSonics has been prepping to bring its Quantra System diagnostic products to market, with prototyping and end-use manufacturing help from Protolabs. In fact, Protolabs has worked with HemoSonics since 2011, from those early R&D days to more recent, end-use production work on the Quantra System.
HemoSonics successfully launched its products commercially last year in Europe, and hopes to enter the U.S. market soon. HemoSonics has expanded its offices into Durham, North Carolina, and has grown to 50-plus employees.
A Need for Speed and Flexibility, Solved by an Agile Supplier
In HemoSonics’ early research and development days, engineers were “iterating through multiple designs under tight deadlines,” said Andy Homyk, senior engineer, who joined the company more than six years ago when it had just five employees. “We were on a tight timeline so we needed a supplier who could machine parts for us quickly, within a couple of days.”
A number of suppliers contacted could not meet those challenging deadlines. Protolabs could, Homyk remembered. “The difference in lead times was dramatic.”
That was in 2012. Since that time, Protolabs has produced hundreds of prototypes and thousands of components for HemoSonics, using 3D printing, CNC machining, injection molding, and sheet metal fabrication for a variety of projects and parts: robotic fixturing, thermal control units, pneumatic manifolds, and more.
“Speed and flexibility—being able to deploy different manufacturing options—and a commitment to customer service, are the main reasons we use Protolabs,” Homyk said.
| At A Glance |
|---|
|
Slash the time of the prototyping process: Engineers at HemoSonics were iterating through designs rapidly under tight deadlines during the company’s early years of R&D, and needed a contract manufacturer that could machine parts quickly. On a more recent project, HemoSonics engineers needed prototype parts about the size of a computer monitor for the casing around the Quantra System blood-clot analysis machine. The prototypes were to demonstrate form, fit, and function of the Quantra System to physicians at various hospitals. Produce custom prototypes for several design iterations within days using quick-turn digital manufacturing. Machining was implemented for some products, and more recently, 3D printing, injection molding, and sheet metal fabrication for the Quantra System products. Engineers also used some finishing options for injection molding, including heat staking and pad printing.
Following years of research and development that included securing key patents, conducting numerous hospital studies, and consulting with physicians and other clinicians, Quantra System products are now on the market in Europe and will launch soon in the United States. |
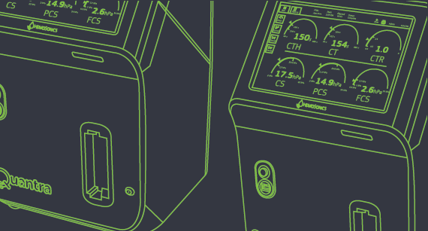
More recently, HemoSonics looked to Protolabs for help with the “skins” or casings that fit around the Quantra System, Homyk explained. HemoSonics engineers needed to design prototypes about the size of a computer monitor—first using 3D printing and then injection molding—to demonstrate form, fit, and function of the Quantra System to physicians at various hospitals.
The Quantra System is designed as a rapid, easy-to-use diagnostic platform that uses disposable cartridges to conduct a panel of tests. The Quantra Hemostasis Analyzer is designed for use in critical care settings that require results to be generated quickly from an instrument that is easy to operate at the point of care.
A challenge emerged when the project switched from additive manufacturing to injection molding. “These are pretty big parts, so one of the molding challenges, in prototyping, was color matching,” Homyk said.

Molding Materials and Finishing Touches
HemoSonics wanted these casings Pantone color-matched to its marketing department’s specifications. One of the ways Protolabs normally does that, in the injection molding process, is to take the plastic resin in the natural color of the specific material chosen, apply around a 3 percent salt-and-pepper mix of colored resins, and final parts are typically very close to the preferred color. But, because of the nature of HemoSonics parts, some swirling and flow marks were showing up on them. “The first batch of parts did not look good cosmetically,” Homyk remembered.
Undaunted, Protolabs went to one of its plastic resin suppliers and the supplier collaborated with Protolabs and HemoSonics to pre-compound the colors. “They mixed the plastic with the dye before molding to get pellets with a nice uniform color,” Homyk said. This custom, pre-colored resin produced flawless parts. “This again speaks to Protolabs’ customer service going the extra mile, and to how agile the company can be.”
Material selection was also carefully considered, Homyk said, given that a requirement of almost any kind of medical device is that it needs to meet certain flammability standards. For the casings, HemoSonics opted for an ABS plastic that met those standards and also offered durability.
Beyond machining, 3D printing, and injection molding, HemoSonics engineers also used some additional finishing options on the injection-molded parts, such as heat staking and pad printing. Heat staking is a process that uses a heated stake to melt metal threaded inserts into plastic parts. This makes it so that screws can be used to attach the Quantra casing parts to a frame, for example.
Pad printing is a process that uses a stamp called a cliche to apply colored logos or decals to parts. HemoSonics used pad printing to put company logos on the Quantra System case parts. Protolabs plans to make these and other finishing options such as mold texturing and part assembly more widely available in the future.

The Outcome? Quantra System Launched in Europe, and is Progressing toward the U.S.
Those long years of research and development, multiple design iterations and prototypes, numerous hospital studies, scores of visits to physicians and other clinicians, the securing of key patents, and the landing of important certifications in Europe—including the CE Mark, is finally paying off, Homyk said. Last year, the Quantra System launched in Europe, and company leaders hope to launch the Quantra System in the U.S.
Going forward, Homyk expects Protolabs to continue to play a key supplier role to support the company’s work. “We pick you guys (Protolabs) because of familiarity, speed of production, flexibility, and exceptional customer service.”
**NOTE: In March, HemoSonics announced that its Quantra Hemostasis Analyzer platform, and its initial QPlus cartridge, have been granted marketing authorization by the FDA and are now available and on the market in the United States.





